article> Science
Vaccines against COVID-19: differently equal
How are the COVID-19 vaccines different and what do all of them have in common?
Originally written for the CEBE blog by Lorena Sánchez
Contributing Writer in collaboration with CEBE

2020 came with a pandemic and loaded with a lot of terms or buzzwords related to the world of vaccines. Never before have we heard people talk as much both in the media and in our daily lives about vaccines, the types of vaccines, and the big pharmaceutical companies and laboratories that develop them. Even so, exposed as we are to so much information, it is not easy to get a clear idea about how the vaccines that have reached the market and are currently being administered to the population work.
What do all COVID-19 vaccines have in common? The answer is simple. Regardless of whether vaccines are of one type or another, they all help our body develop immunity against the virus that causes the disease by generating, on the one hand, antibodies that recognize and fight it, and on the other, memory cells. The latter will quickly notify our body that it has encountered the same virus again, in which case antibodies will be produced again to attack it. To date, one of the great unknowns that is being studied is how long these memory cells can protect us against the virus.
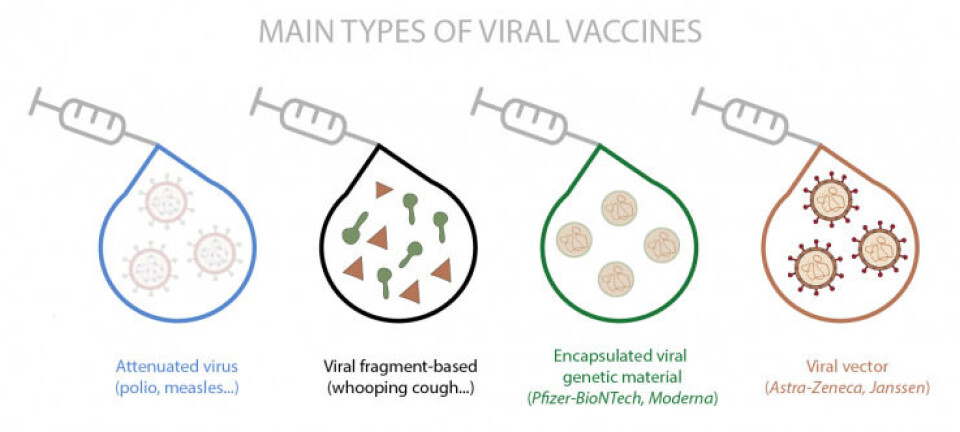
There are different types of vaccines currently approved (and in use) and others that are in the later stages of clinical trials. In fact, it is the first time that so many vaccine candidates are being developed simultaneously against the same disease.
There are different methods of making vaccines. The most traditional method uses the whole virus, either inactivated or attenuated (such as polio and measles, respectively) or fragments of it (such as whooping cough). There are other slightly more novel methods, in which a harmless empty virus (viral vector) is used that carries either the genes that express proteins or the parts of the proteins that belong to the virus against which it is intended to create immunity. And other completely new methods include those that incorporate encapsulation of the genetic material that contains the instructions for making virus-specific proteins. This last type includes the first vaccines approved for use in humans, the so-called messenger RNA (mRNA) vaccines, which are those from Pfizer-BioNTech and Moderna.
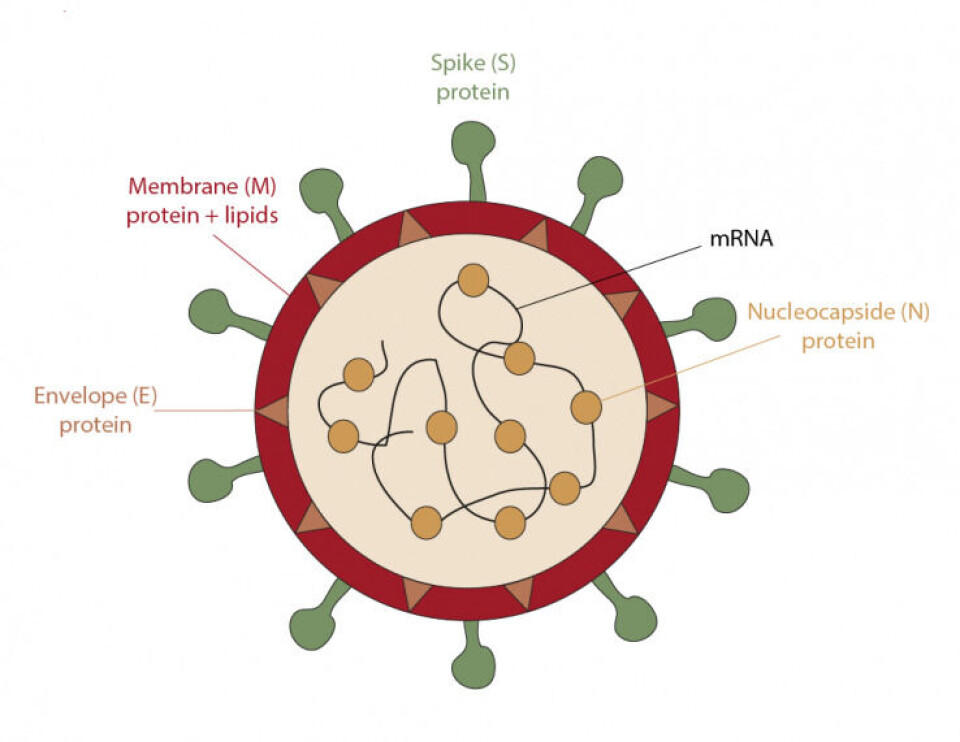
Both vaccines are quite similar since they both contain the information necessary to synthesize the main antigen of the coronavirus, which is the spike protein (S), found on the surface of the virus. To put it in other words, this protein is like the key that the virus uses to enter the cells of our body. Once one of these vaccines is administered, the mRNA enters the cells and serves as a template to generate copies of the S protein. After this process, the cell removes the mRNA. Meanwhile, the manufactured proteins are transported to the surface of the cell, or fragmented into small pieces that also appear on the surface, and in this way are recognized by the immune system, generating antibodies and memory cells. In case, if someone is infected after the administration of a vaccine, the body recognizes the virus and fights it. The two vaccines require two doses to increase their effectiveness, although the time between the two inoculations is different. Pfizer's requires 21 days between doses and Moderna's 28. The main disadvantage of these vaccines is their transport and storage, as it requires a permanent cold chain with extremely low temperatures that require freezers of -20 and -80 degree centigrade.
The next vaccine to be approved in the European Union was from the pharmaceutical company AstraZeneca. This vaccine uses an inactivated adenovirus, that is, a virus that cannot divide within our body, as a vector to introduce the genetic material necessary for our cells to generate the coronavirus spike protein, and thus our immune system responds by creating antibodies and memory cells against SARS-CoV-2. Like the previous ones, this vaccine requires two doses to be more effective, and both must be between 10 and 12 weeks apart (preferably 12). This vaccine is transported and stored between 2 and 8 degrees Celsius, which facilitates better distribution compared to those mentioned above. Another vaccine of the same type has recently been approved in the United States and Canada developed by the Belgian company Janssen, and it also uses a similar vector (inactivated adenovirus) and which, unlike AstraZeneca's, requires a single dose. Its possible use is currently being evaluated in the European Union.
In addition to those already mentioned, there are a large number of candidates still under development and evaluation that we will be hearing about throughout this year. In the same way, also during this year, we will see if these vaccines, as they are now, are enough to combat the new variants or if, on the contrary, they will need to be modified or adapted. In any case, all of these platforms would allow these necessary changes to be made with relative ease.
For more regular content
- Follow us on Facebook: https://www.facebook.com/thevoice.loko
- Check out our Instagram page: https://www.instagram.com/thevoice.kuleuven/
- Listen to our podcasts on: https://www.mixcloud.com/The_Voice_KUL_Student_Radio
For submissions or applications
- Email us at thevoice@loko.be
- Or message us on Facebook





